Berkeley Advanced Biomaterials (BAB) specializes in developing and manufacturing hydroxyapatite (HAP), tricalcium phosphate (TCP), and other calcium-based products.

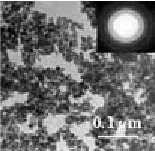
Our standard HAP powder has an average diameter of 5 µm. The size distribution is narrow and follows a normal distribution. The individual micro-spheres are porous with an average pore size of 100 nm. The size and morphology of the powder make it suitable for high-resolution liquid chromatography.
Other powder sizes are available (less than 150 µm). Denser spherical powder and donut-shaped powder can also be produced upon special request. For more technical information, please call us or send us mail.

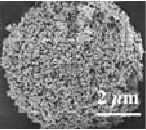

Pure hydroxyapatite granules come in six standard sizes: 10-50 µm, 50-150 µm, 100-300 µm, 500-1000 µm, 1-3 mm and 3-6 mm. They are either dense or porous. The granules are suitable for chromatography as well as bone refill and drug delivery systems research applications.
Pure Tri-calcium Phosphate granules come in six standard sizes: 10-50 µm, 50-150 µm, 100-300 µm, 500-1000 µm, 1-3 mm and 3-6 mm. They are either dense or porous. The granules are suitable for chromatography as well as bone refill and drug delivery systems research applications.
Bi-phasic (HAP-TCP) granules are available upon request. We can also incorporate water-soluble drugs homogeneously in the granules according to customer’s specifications(see our applications page).

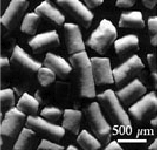
Research and Development products are “Medical Grade”, non-sterile and not approved for human use. BAB does not provide certificates of analysis for research products. USP does not have a monograph describing HAP nor Beta-TCP. Medical Grade hydroxyapatite conforms to ASTM Standard F1185-03, and Beta Tricalcium Phosphate conforms to ASTM F1088-04a.
Morphology:
Agglomerated needle-shaped dry powder.
Typical Usage:
Use as an ingredient to make composites, for DNA and protein purification, or as reference material.
BABI-HAP-N100
Hydroxyapatite (dry)
BABI-TCP-N100
Tri-calcium Phosphate
Particle size:
Avg. 100 nm
Specific Surface Area:
50-70 m2/g
Morphology:
Agglomerated sphere-like powder.
Pore size:
< 0.5 μm.
BABI-HAP-SP
Hydroxyapatite
BABI-TCP-SP
Tri-calcium Phosphate
Particle size:
Avg. 5 μm
Specific Surface Area:
10 m2/g
Morphology:
Granules.
BABI-HAP-G2
Hydroxyapatite
BABI-TCP-G2
Tri-calcium Phosphate
BABI-HATCP-G2
Hydroxyapatite and Tri-calcium Phosphate
Particle size:
50-150 μm
 During the last two decades, significant progress has been made in the area of drug delivery systems. Drug delivery in the future will require sophisticated devices that are controlled, self-regulated, and can release drugs only at target sites. With the recent developments in micro-encapsulation technology, HAP micro-spheres are being considered for drug delivery applications. HAP has the advantage of being bio-compatible, bio-resorbable, and high binding to a variety of molecules (e.g. proteins, enzymes, antibody fragments, nucleic acids, and some subclasses of IgG). This has opened the potential for using HAP to deliver a large variety of drugs in many clinical applications.
During the last two decades, significant progress has been made in the area of drug delivery systems. Drug delivery in the future will require sophisticated devices that are controlled, self-regulated, and can release drugs only at target sites. With the recent developments in micro-encapsulation technology, HAP micro-spheres are being considered for drug delivery applications. HAP has the advantage of being bio-compatible, bio-resorbable, and high binding to a variety of molecules (e.g. proteins, enzymes, antibody fragments, nucleic acids, and some subclasses of IgG). This has opened the potential for using HAP to deliver a large variety of drugs in many clinical applications.
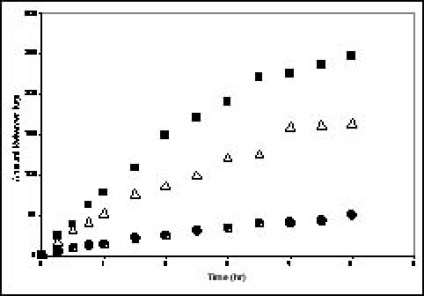
A study of HAP implants for the treatment of bone infections was recently performed in collaboration with Professor A. K. Dash from theDepartment of Pharmaceutical and Administrative Sciences at Creighton University. HAP microspheres containing ciprofloxacin were prepared by precipitation followed by a spray drying process. The drug load in the micro-spheres ranged from 0.25-2% (w/w). SEM, powder X-ray diffraction, and Differential Scanning Calorimetry showed that the surface morphology of the micro-spheres was rough and that the drug was present at a molecularly dispersed state in the HAP. Implants (5×8 mm) were made by pressing the powder into small cylinders. The mechanism of in vitro drug release from the implant was found to be controlled by diffusion in the matrix. For this set of experiments, complete release of the drug occurred within 10 hours (see Fig. 1). The release rate can be increased by increasing the concentration of the drug and decreased by introducing diffusion barriers in the implants. Further information on this study can be found in “Preparation and characterization of novel drug delivery implants for the treatment of bone infections”, by H.H. Pham, P. Luo, F. Génin, and A. K. Dash, Proceedings of the AAPS Meeting (New Orleans, November 1999).
Figure 1: In vitro release of ciprofloxacin from HAP implants containing;() 2%
(w/w),() 1% (w/w), and(•) 0.5% (w/w) of theoretical drug load.
Calcium phosphate apatite (CPA) is known to be one of the most important implantable materials due to its biocompatibility. Natural bone is approximately 70% CPA including hydroxyapatite (HAP) by weight and 50% by volume. Organic substances (such as collagen) and water are the other constituents of bones. The CPA mineral is nanocrystalline with grains less than 50 nm. These nanocrystals are connected to each other to form the connective hard tissue, (i.e. the bone skeleton).
Bone is a complex structure with macro- and micro-pores. The pores are mostly interconnected to allow body fluid to carry nutrients and provide a medium where interfacial reactions between hard tissue and soft tissue can occur. Two types of bone structures have been described: cancellous and cortical structures. Cancellous bone differs from cortical bone in being open-spaced and trabecular. Since osteons average between 180 and 250 µm in diameter and intercommunicate through Volksmann canals, the sizeof the interconnected pores have similar dimensions.
Implant materials must provide properties that are similar to that of bone. Currently, only natural materials have been selected as bone substitute for bone grafting surgery. These include auto- or allograft, bovine, and coral blocks. Although these materials can closely replicate the structure of human bone, they present a number of disadvantages. They are typically very brittle and experience significant loss of strength during sterilization. They also can cause inflammation problems and carry the potential for disease transmission.
To address these issues, our research is focussing in developing high strength synthetic biomaterials such as CPA and HAP ceramics. The ceramics are either sintered porous, or dense calcium-based mixtures (including HAP and TCP). Our research team recently completed an extensive characterization of the sintering behavior of our narrow size distribution powder. We also have completed a study of the phase transformations that can occur in our sintered micro-porous HAP. Our research team is now designinghigh strength three-dimensional cellular biomaterials.
Ping Luo, Nan Liu, and Michael Thelen, “Protein binding potential of biocompatible ceramic hydroxyapatite micro-spheres”, 24th Annual Meeting of the Society for Biomaterials, Vol. 21, 278 (1998).
Hai H. Pham, Ping Luo, François Génin, and Alekha K. Dash, ” Preparation and characterization of hydroxyapatite microspheres containing ciprofloxacin”, AAPS Annual Meeting, New Orleans, November 14-18 1999.
Luo, F. Y. Génin, and T. G. Nieh, Synthetic calcium phsophate-based skeletons, Proceedings of the Fifth World Biomaterials Congress, Toronto, 627 (1996).
Ping Luo, “Methods of Treating NuclearHydroxyapatite Materials”, US Patent 5994609, Nov.1999
Ping Luo, “Method of Synthesizing HydroxyapatitePowders and Bulk Materials”, US patent 5858318, Jan.1999